DNA-Carbon Dot Hybrid Hydrogel for Sustained Release of Cancer Drug
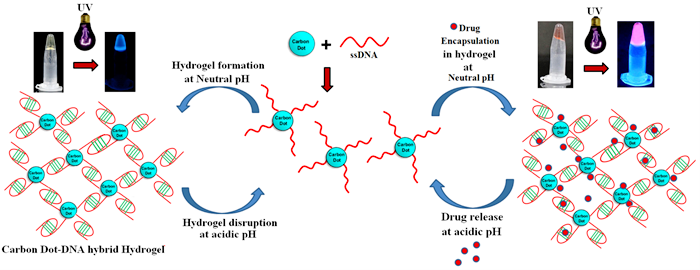
The construction of DNA-carbon dot (CD) hybrid hydrogel for targeted and sustained release of drug molecules has been achieved for the first time. Amine functionalized carbon dots were conjugated to 5’-phosphate termini of Cytosine (C) rich ssDNA. As a prototype, chemotherapeutic drug Doxorubicin (Dox) was loaded and enclosed in hydrogel that acts as a container for sustained release of the drug. The photophysical properties of CD potentially enable tracking of hydrogel dissolution and drug cargo loading in hydrogel. The visually detectable sol-gel transition of CD-DNA hybrid hydrogel was achieved by varying the pH of the solution from alkaline to neutral. The in vitro time and pH dependent release profile of the drug from hydrogel show significant sustained release of the drug. While hydrogel was found to be stable for a month at normal physiological pH, complete dissolution and sustained release of the drug molecules were achieved over 10-11 days in acidic pH that is relevant to tumor microenvironment. The cell viability assay performed on HeLa cells shows their effective slow killing in presence of the Dox loaded hybrid hydrogel owing to favorable acidic pH for hydrogel disruption. This has been reported in the Journal “CARBON” (Carbon dots assisted formation of DNA hydrogel for sustained release of drug, Seema Singh, Anshul Mishra, Rina Kumari, Kislay K. Sinha, Manoj K. Singh and Prolay Das, Carbon, 2017, 114, 169-176).
http://www.sciencedirect.com/science/article/pii/S0008622316310971