Research Areas
Process Systems Engineering Laboratory (PSEL)
The process industries are among the most important manufacturing facilities. They cover a broad array of industries, including chemical, cement, iron and steel, petroleum, gas, petrochemical, pharmaceutical, food, microelectronics, metal, textile, and forestry products. The efficiency of these industries is strongly dependent on their engineering. Process Systems Engineering laboratory (PSEL) deals in aspects of planning, design and formation of sustainable process and product supply chain along with optimal and efficient operation of process systems. PSEL is continuously working toward the objective of
developing innovative process and systems engineering solutions to contribute towards universal sustainability and prosperity. PSEL aims to develop process and product design with emphasis on conservation of natural resources; in particular, materials, energy and water. The research works carried out in PSEL have been published in premiere international journals and also been presented in reputed international conferences.
Gas-Solid Interaction Laboratory (GSIL)
At GSIL, our research efforts are primarily directed in exploring novel catalysts/process routes for clean energy applications. Currently, the major area of our research are as follows:
1.Solar-to-fuel conversion:
It is a belief that one of the prime reasons for global warming is ever-increasing amount of CO2 into the atmosphere. Here, we focus on converting CO2to fuels (such as CH4, CO+H2, CH3OH) using an
“efficient” photocatalystsin the presence of sunlight. Specifically, we aim to identify and thus increase yield, and selectivity of the reaction products. Moreover, our laboratory studies the fundamental aspects of such photocatalytic reaction.
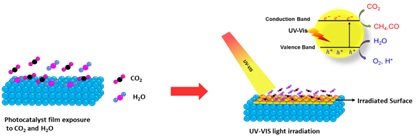
Students: Mr. Niwesh Ojha (doctoral student) and Mr. Abhinav Bajpai (doctoral student)
2.Ammonia synthesis
Iron and ruthenium-based catalysts are the two main catalyst system currently in use to produce ammonia, but at very high pressure (~100-150bar) and temperature (400-500°C). Our laboratory intent to explore suitable catalyst that can lower the required pressure to merely 1bar. We are also interested in gaining insight to the behavior of nitrogen and hydrogen gaseous molecules with the chosen catalyst.
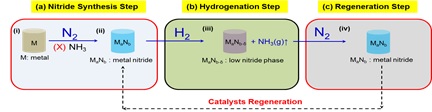
Students: Mr. Pintu Kumar Roy (JRF)
3. Heterogeneous catalysis:
Several other industrially significant processes such as Sabatier reaction, Dry reforming of methane involves conversion of CO2 to valuable fuels. Here, we aim to explore novel catalysts which can be more effective in mild condition. Additionally, we focus to understand role of other controlling factors, which can substantially affect the reaction.
Students: Mr. Abhinav Bajpai (doctoral student)
Chemical Process Development Laboratory (CPDL)
Chemical engineers have always strived towards improving energy, material, and cost efficiency in processes. However, modern industrial processes focus not only on their efficiency, but also on the environmental impact. With this motivation, we aim at developing novel processes for production and separation of chemicals with improved efficiency while ensuring sustainability. Process development aims at integrating several aspects of chemical engineering to improve or develop a process. The processes range from simple filtration to complex fermentation and downstream processing.
CPDL focusses on the practical aspects of improving and establishing a process for a specific objective. The current research areas include the following:
i.Development of a continuous separation process complementing continuous chemical synthesis.
ii.Development of downstream processes for fermentation based products.
iii.Study of ayurvedic manufacturing process for streamlining and improving reproducibility in an industrial scale.
Development of an effective process for biodiesel production from waste resources.
Energy &Thermofluids Laboratory (E-Therm)
E-Therm Lab focuses on solving emerging and cutting edge problems in fluid, energy and particle transport systems such as duct flows, flow past submerged surfaces, convective heat and mass transport in non-Newtonian fluids (power-law, viscoplastics, viscoelastics), rheologyof smart fluids (ER, MR), motion of bubble and drops, thermal energy storage using microencapsulated phase change materials (MPCMs), two-way coupled multiphase flow behavior, flow and deposition of ultrafine particle in arteries, flow through porous media, etc., using CFD tools such as COMSOL Multiphysics, ANSYS Fluent, and OpenFOAM to delineate the influence of pertinent governing parameters on momentumand heat/mass transfer characteristics.
(Research motivated students with good academic record are encouraged to join us.)
Active areas of research
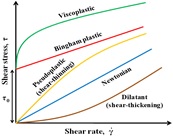
- Internal/external boundary layer flows in non-Newtonian media
Unlike the case of the Newtonian fluids, which are characterized by a constant shear viscosity, most of the structured fluids such as polymers melts, suspensions, emulsions and foams, worm-like micellar solutions, etc., often display non-Newtonian characteristics such as shear-dependent viscosity. Another class of non-Newtonian fluids is the viscoplastics such as Bingham plastics, Herschel-Bulkley fluids, etc. (for example, toothpaste, chocolate, butter, jam, cosmetics, mortars, foams, muds, mayonnaise, etc.) which are characterized by the existence of a yield stress below which the fluid does not shear. Depending upon the magnitude of fluid yield stress, such materials may behave like a fluid or a solid. This distinct feature of such materials is used to augment the rate of heat transfer (in forced- and mixed-convection) in the systems dealing with the stability of suspensions, heating/cooling of temperature-sensitive materials, cyclone separators, gravity settlers, etc.
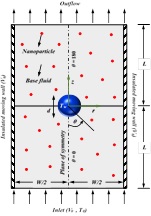
- Heat transfer augmentation in nanofluids
Over the years, several attempts have been made to increase the effective thermal conductivity of the process fluids. For instance, the use of nanoparticles (NPs) of size
- Utilizing PCMs for efficient thermal energy storage
Due to limited reserves of non-renewable resources such as coal, oil and gas, nuclear substances, etc., it becomes highly important to search for an ecofriendly and cost-effective technology for energy storage. The thermal energy storage (TES) systems play a vital role in HVAC, solar equipment, refrigeration, waste heat recovery, green house, smart buildings, textiles, thermal comfort in vehicles, etc. In recent years, phase change materials (PCM) have attracted the major attention among researchers due to its high energy storage density in form of the latent heat of fusion at a constant phase transition temperature. This attractive property of PCM improves the thermal comfort and reduce the energy consumption for heating/cooling purposes.
- Coupled CFD-DPM/CFD-DEM simulations
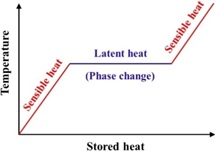
We carry out numerical simulations for lean (conc. < 10%) and dense (conc. > 10%) particle systems related to aerosol dynamics, fluidized beds, particle-particle and particle-wall interactions (both Eulerian and Lagrangian), agglomeration and segregation of particulate matter associated with environmental chemical engineering, etc.
Modeling, Analysis and INformatics Laboratory for Energy Systems (MAINLABES)
Contact Person: Dr. Ashwini Kumar Sharma, Email: ashwinikumar@iitp.ac.in
In view of the unsustainability and negative environmental impact of current fossil fuel-based economy, renewable-energy sources, such as solar and wind, are being deployed in larger numbers than ever before. Considering the limited efficiency, unpredictability and intermittency of these renewable sources, there is a quest for scalable and efficient energy conversion and storage (ECS) technologies. Electrochemical systems in the form of fuel cells, batteries and flow batteries have emerged as prime candidates in this regard. Although the necessary infrastructure for the renewable energy has not been fully developed yet, these emerging energy systems have received considerable attention. In addition to their inherent advantages, further research on their system and component design is a key to contend and replace the traditional counterparts of internal combustion engines.
Research program in MAINLABES relies heavily on advanced mathematical modeling, analysis of transport phenomena, and data science techniques with focus on design and development of emerging and sustainable energy systems in particular fuel cells, batteries and flow batteries. The overall aimis to derive solutions to support the commercialization of these systems based on mathematical modeling and simulations. Simulating these systems with sufficiently high accuracy and fidelity can not only accelerate the development and design process but also do so at a significantly lower cost. Fundamental research in the lab can be characterized by estimating and understanding the physical phenomena a priori to computations, followed by reductions in the mathematical formulation without sacrificing any of the essential physics and geometrical resolution. This approach allows for a deeper understanding as compared to only solving the model numerically for various parameter changes. In the applied research, we are working on application specific problems for different systems, e.g., state estimation, thermal management, fast charging, etc. for lithium-ion batteries.

Computational Nanoscience Laboratory (CNSL)
We are interested to understand the structural, dynamical and Interfacial properties of complex fluids at the nanoscale. We use Monte Carlo method, Molecular Dynamic Simulation and Density Functional Theory to probe the properties of fluids at molecular level.
Research Focus:
1.Surface Phase transition of polar molecules.
2.Development of Novel Materials like super-hydrophobic, super-oleophobic, anti-fouling, anti-icing
surfaces etc.
3.Self-Assembled Monolayer (SAMs) in application of chemical sensor.
4.Thermo-physical properties of actinides compounds in application of nuclear fuel.
5.Properties of Confined fluids.

Biochemical Laboratory
Our research group is primarily working on advanced water treatment technologies that include various advanced oxidation processes (AOPs) such as heterogeneous and homogeneous photocatalysis, photo-independent processes, etc. In addition to that, we are also working on the enhancement of the effectiveness of existing AOPs and adsorbents. For the same, we have been experimenting with some hybrid combinations such as microwave and/or ultrasound irradiation assisted AOPs, and microwave enhanced or induced adsorption processes. Moreover, our research group is also working on rapid, economical and green synthesis of novel nanoparticles, which are supposed to be used as potential catalysts and/or adsorbents.
On the other hand, this group is also working on the effective utilization of microwave irradiation for warming of heat sensitive bio-materials. The aim is to reduce the processing time efficiently without compromising with the quality or nature of the heating substance. Currently the theoretical analysis is being carried out using COMSOL and in-house codes. Also, we have 'Vector Network Analyzer' with co-axial mode for the measurement of dielectric properties of various samples (such as liquids, solids, and semi-solid samples).
Facilities Available:
- V-Visible Spectroscopy (Shimadzu UV-2600)
- Diffuse Reflectance Spectroscopy (Shimadzu UV-2600)
- Digital Photo Reaction System (NSGI, Hyderabad)
- Ultrasonic Microwave Reaction System with UV Irradiation (Nanjing Xianou Instruments Manufacture Co., Ltd, China; XO-SM100)
- Vector Network Analyzer (Keysight ENA Vector Network Analyzer – E5071C)
- LC-MS/MS (Agilent Series Model 6545 Q-TOF LC-MS)
- Other Wet Lab Facilities (Including Fume Hood, Biosafety Cabinet, Hot Air Oven, Incubator, Autoclave, Centrifuge, Ultrasonic Bath, Probe Sonicator, Freezer, Microwave Oven, Water Distillation Unit, etc.)
Ph.D Research Scholars:
Mr. Priyanshu Verma (Thesis Submitted);
Ms. Sandhya Mishra;
Ms. Sushma Kumari
Research Highlights:
1.UV/TiO2 based Heterogeneous Photocatalysis for Degradation of Mixed Pollutants

Verma, P.; Samanta, S. K. Res. Chem. Intermed. 2017, 43, 6317–6341
(https://doi.org/10.1007/s11164-017-2992-6)
2.Enhancement of UV/TiO2 based Degradation Performance
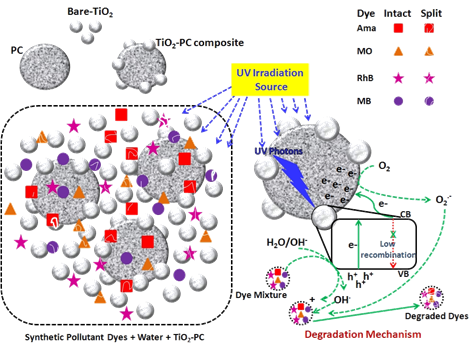
Verma, P.; Samanta, S. K. Res. Chem. Intermed. 2018, 44, 1963–1988
(https://doi.org/10.1007/s11164-017-3209-8)
3.Microwave-enhanced Advanced Oxidation Processes for the Degradation of Dyes
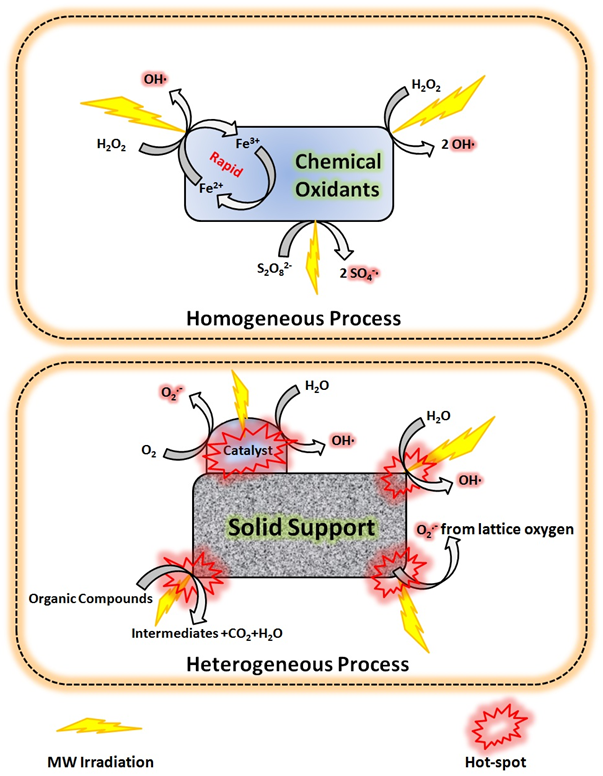
Verma, P.; Samanta, S. K. Environ. Chem. Lett. 2018, 16, 969–1007
(https://doi.org/10.1007/s10311-018-0739-2)
4.A Direct Method to Determine the Adsorbed Dyes on Adsorbent via Processing of DRS Data
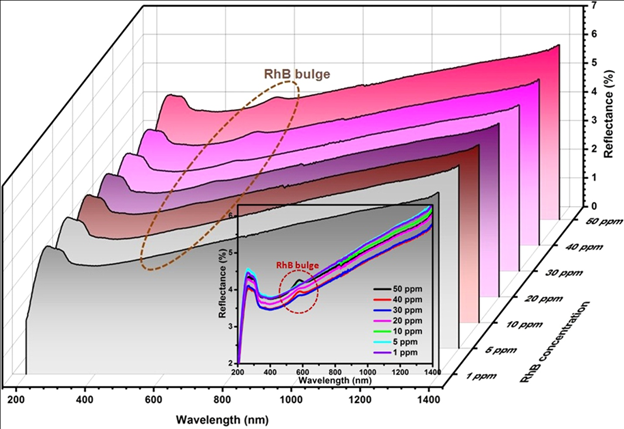
Verma, P.; Samanta, S. K. Mater. Res. Express 2019, 6, 015505
(https://doi.org/10.1088/2053-1591/aae3f2)