Condensation of DNA- A Putative Obstruction for Repair Process in Abasic Clustered DNA Damage
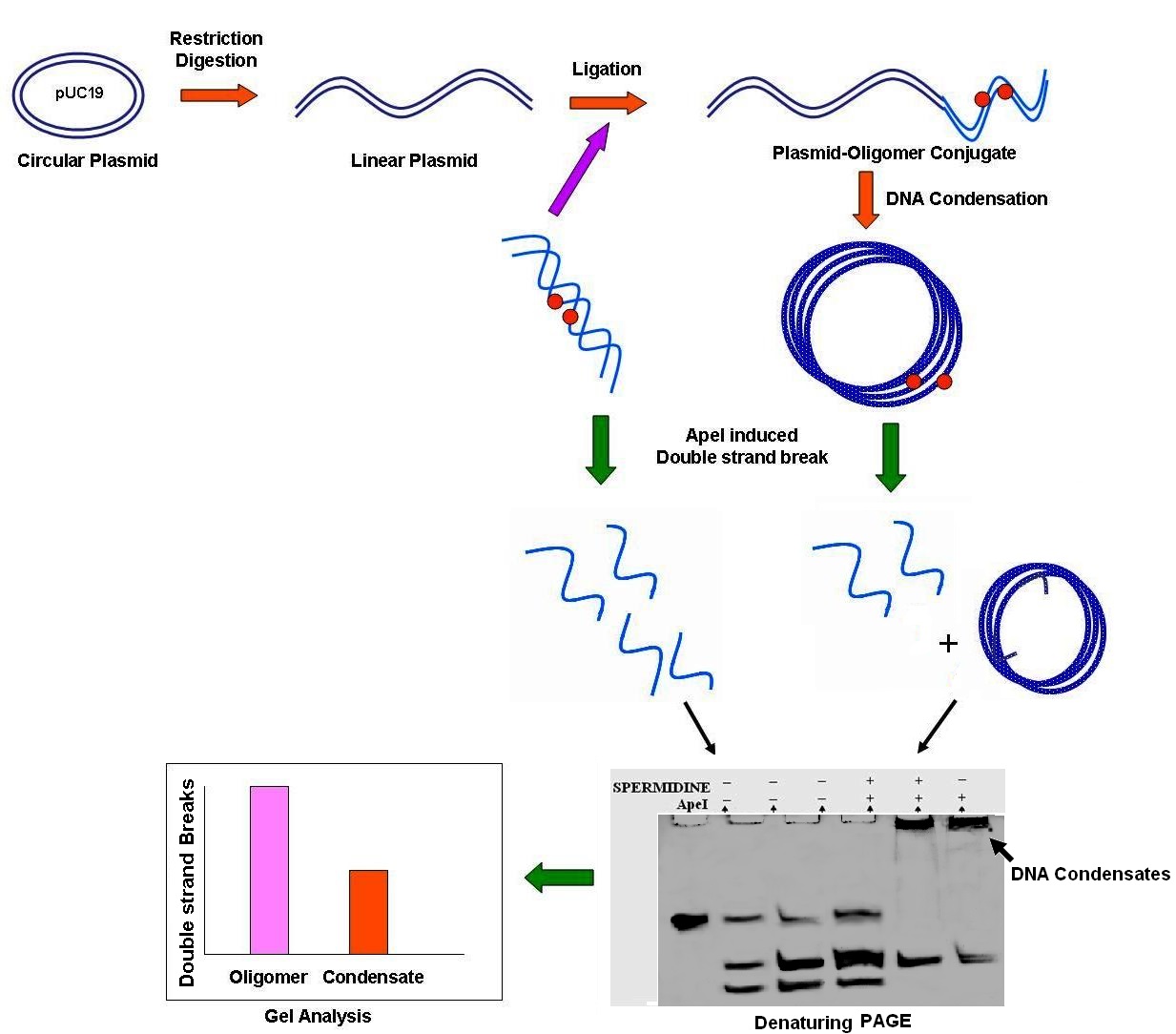
Clustered DNA damages are defined as two or more closely located DNA damage lesions that may be present within a few helical turns of the DNA double strand. These damages are potential signatures of ionizing radiation and are often found to be repair resistant. Types of damaged lesions frequently found inside clustered DNA damage sites include oxidized bases, abasic sites, nucleotide dimers, strand breaks or their complex combinations. In this study, we used a bistranded two-lesion abasic cluster DNA damage model to access the repair process of DNA in condensate form.
Oligomer DNA duplexes (47 bp) were designed to have two deoxyuridine in the middle of the sequences, three bases apart in opposite strands. The deoxyuridine residues were converted into abasic sites by treatment with UDG enzyme creating an abasic clustered damage site in a precise position in each of the single strand of the DNA duplex. This oligomer duplex having compatible cohesive ends was ligated to pUC19 plasmid, linearized with HindIII restriction endonuclease. The plasmid-oligomer conjugate was transformed into condensates by treating them with spermidine. The efficiency of strand cleavage action of ApeI enzyme on the abasic sites was determined by denaturing PAGE after timed incubation of the oligomer duplex and the oligomer-plasmid conjugate in presence and absence of spermidine. The efficiency of double strand breaks was determined similarly by native PAGE. Quantitative gel analysis revealed that rate of abasic site cleavage is reduced in the DNA condensates as compared to the oligomer DNA duplex or the linear ligated oligomer-plasmid conjugates. Generation of double strand break is significantly reduced also, suggesting that their creation is not proportionate to the number of abasic sites cleaved in the condensate model. All these suggest that the ApeI enzyme have difficulty to access the abasic sites located deep into the condensates leading to repair refractivity of the damages. In addition, it was found that presence of a polyamine like spermidine has no notable effect in the incision activity of ApeI enzyme in linear oligomer DNA duplexes in our experimental concentration. These findings were published in the Journal “DNA Repair” authored by Vandana Singh and Prolay Das. (http://dx.doi.org/10.1016/j.dnarep.2013.03.002)