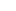Binding and interaction of di- and tri-substituted organometallic triptycene palladium complexes with DNA
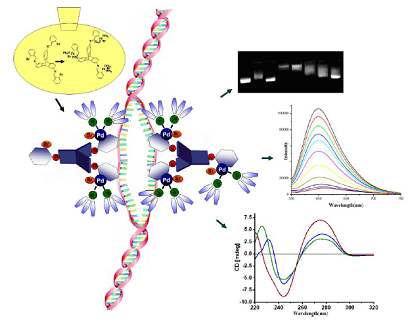
Two triptycene-based ligands with pendant bromophenyl units have been prepared and used as synthons for the synthesis of di and tri nuclear palladium complexes. The organic molecules and their corresponding organometallic complexes have been fully characterized using nuclear magnetic resonance (NMR), infrared (IR) spectroscopy and mass spectrometry. The mode of binding and effect of the complexes on pUC19 plasmid, calf thymus DNA and oligomer duplex DNA have been investigated by a host of analytical methods. The complexes brought about unwinding of supercoiled plasmid and the unwinding angle was found to be related to the binding affinity of the complexes with DNA, where both these parameters were guided by the structure of the complexes. Concentration dependent inhibition of endonuclease activity of by the complexes indicates preference for G/C sequence for binding to DNA. However, neither the complexes did not introduce any cleavage at abasic site in oligomer duplex DNA, nor they created linear form of the plasmid upon co-incubation with the DNA samples. The interactions of the complexes with DNA were found to be strongly guided by the structure of the complexes, where intercalation as well as groove binding was observed, without inflicting any damage to the DNA. The mode of interaction of the complexes with DNA was further confirmed by isothermal calorimetry. This is reported in the Journal of Biological Inorganic Chemistry (2014, DOI 10.1007/s00775-014-1180-z) by Dr. Prolay Das and Dr. Neeladri Das and their research groups.